Life Sciences: Pharmaceuticals & Medical Devices
Secure your supply chain with the industry’s longest-tenured track and trace solutions.
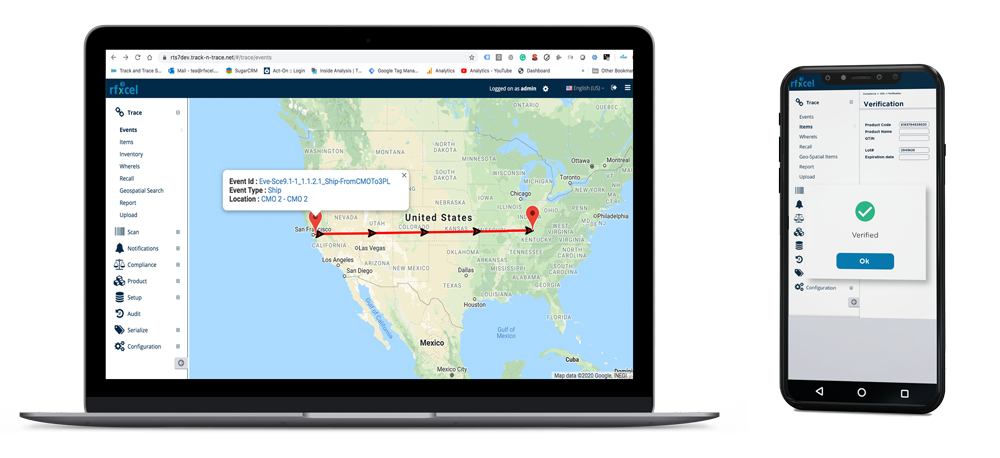
Popular Features
- Raw ingredients to finished goods traceability
- Real-time cold chain monitoring
- Compliance management
- Predictive analytics
Teams of experts implement and support your solution
Full-service implementation and validation
- With thousands of integrations completed, we walk you through every step of the process to ensure your supply chain is optimized.
- Pre-configured and pre-validated solutions simplify your implementation process.
- Receive documented release notes, risk assessments, and detailed test results.
- A fully validated production solution can be implemented in 20–30 days.
Advisory services and support
- We offer 24/7 support to all our customers.
- You are assigned a dedicated support person to address all your inquiries and needs.
- We believe in educating industry players to maximize interoperability.
200,000+
successful Integrations and connections for customers and their partners
How rfxcel is leading the digital supply chain for the Life Sciences
Secure your supply chain with rfxcel
Our track and trace products help patients get the right drug at the right time, combat counterfeit drugs, and secure your business investments.
View more rfxcel videos for Life Sciences
See how customers are succeeding with rfxcel

DCC Vital selects rfxcel as a serialisation solution provider
rfxcel, the longest-tenured track and trace solution provider in the life sciences industry, has been selected by DCC Vital, a manufacturer, to meet its European Falsified Medicines Directive (EU FMD) serialisation requirements.

Abdi Ibrahim selects rfxcel for EU FMD compliance
Abdi Ibrahim, the Turkish pharmaceutical industry leader, has selected rfxcel to implement and support new compliance management and serialisation processing software to ensure full compliance with current and future international legislative requirements ahead of the 2019 EU Falsified Medicines Directive (FMD) deadline.