LEADING SUPPLY CHAIN TRACEABILITY SOLUTION
- Reduce Cost
- Eliminate Complexity
- Improve Visibility
- Enhance Control
- Ensure Compliance
rfxcel Is Now Antares Vision Group Supply Chain Visibility
Delivering a supply chain solution with real-time data, end-to-end traceability, environmental monitoring, and compliance. Explore what we can do for you.

Empowering our customers to protect product, profit, people, and planet.
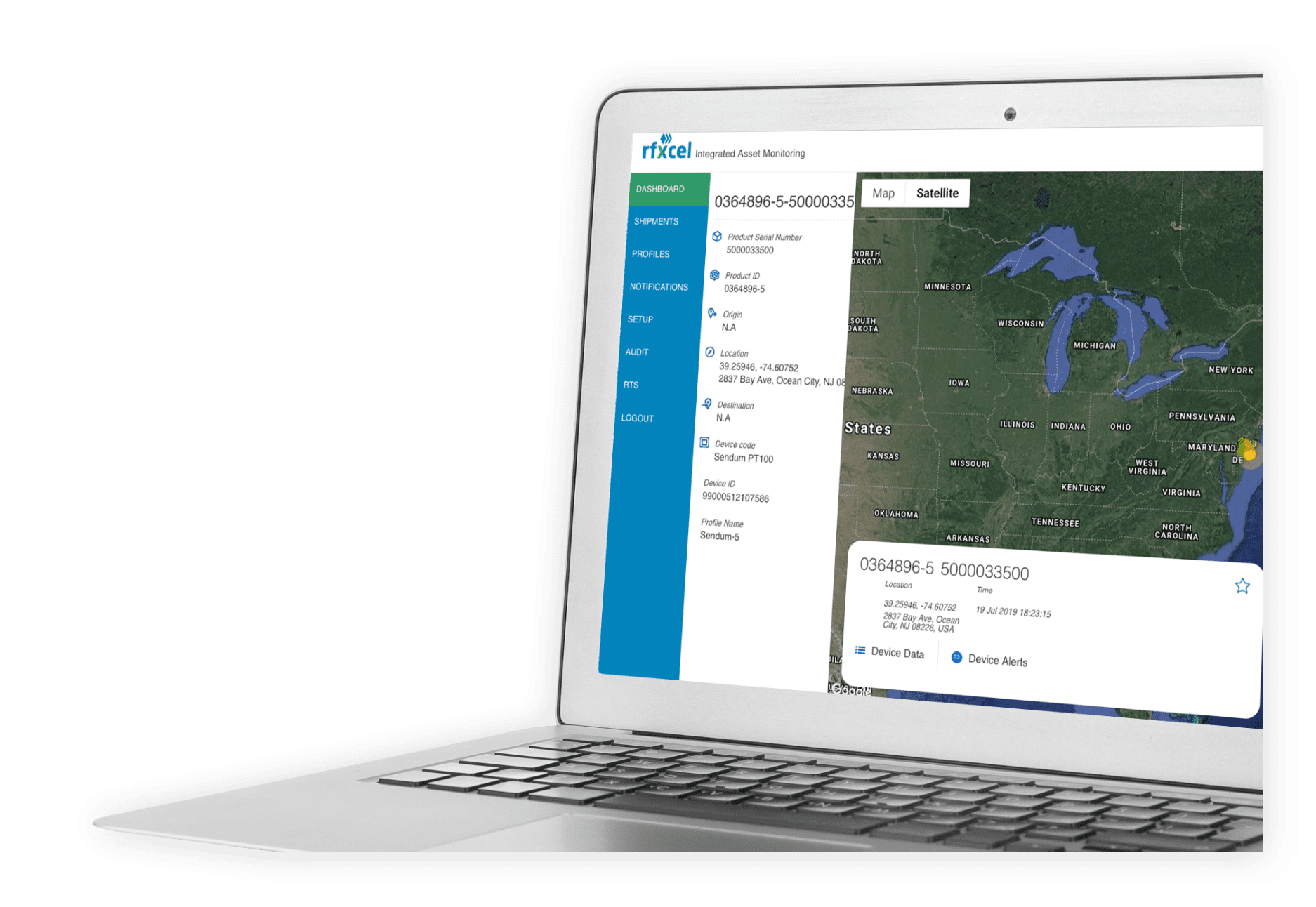
Complete Supply Chain Visibility & Security
Deliver the right product to the right place at the right time with total visibility along the way.

Protect your supply chain with rfxcel Traceability System.
rfxcel Track and Trace Solutions
See how customers are succeeding with rfxcel
Ready to see what rfxcel can do?
Latest from rfxcel

The Drug Quality and Security Act (DQSA) and the Drug Supply Chain Security Act (DSCSA) forever changed pharmaceutical warehouse management.…

The Food and Drug Administration’s (FDA) Food Safety Modernization Act completely changed how members of the food manufacturing supply chain…
Indonesia track and trace regulations are designed to prevent counterfeit, stolen, contaminated, or otherwise harmful drugs from entering the supply…

The Food Safety and Modernization Act (FSMA) is going to have a major impact on the food industry. FSMA gives…