Regulatory Compliance Software
MEET GLOBAL PHARMACEUTICAL COMPLIANCE REQUIREMENTS WITH
RFXCEL’S COMPLIANCE MANAGEMENT SOFTWARE
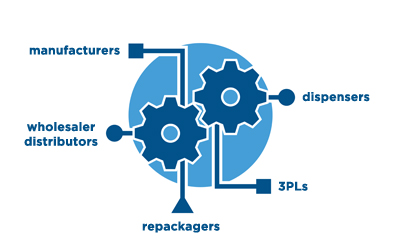
Compliance often requires a significant investment and a change in business processes. With the demands placed on pharmaceutical companies to safeguard the supply chain from theft, diversion, and counterfeiting, we believe that you should gain additional value and insights from your software compliance investment. At rfxcel, we don’t just think of compliance software as a way to check the box, we use compliance software as a vehicle to catapult our customers’ organizations into a new level of supply chain productivity.