2026 India User Forum - Strengthening Compliance and Driving Results
Join us this March for exclusive in-person sessions designed to help you maximize compliance, efficiency, and performance in 2026. March 17–20, Mumbai, Ahmedabad, Hyderabad.
- Strategic compliance discussions
- Direct product feedback & roadmap insight
- Practical takeaways you can apply immediately
Introducing DIAMIND Sentry: The Next Generation of Error Handling
Delivering a supply chain solution with real-time data, end-to-end traceability, environmental monitoring, and compliance. Explore what we can do for you.

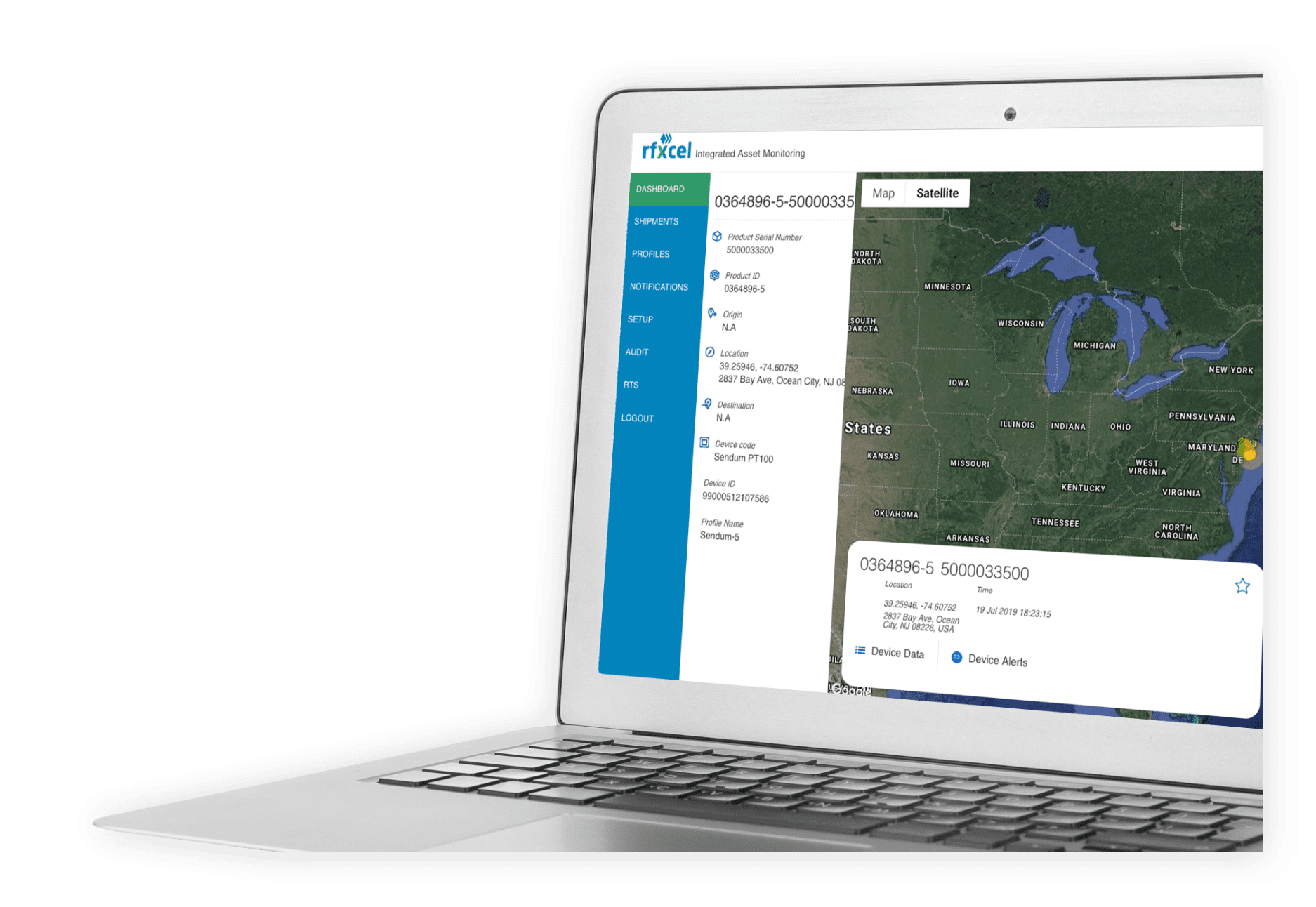
Empowering our customers to protect product, profit, people, and planet.
Complete Supply Chain Visibility & Security
Deliver the right product to the right place at the right time with total visibility along the way.

Protect your supply chain with rfxcel Traceability System.
rfxcel Track and Trace Solutions
See how customers are succeeding with rfxcel
Ready to see what rfxcel can do?
Latest from rfxcel

New York, NY – November, 2025 – Antares Vision Group - a global leader in traceability and quality…

A LinkedIn Live Recap Featuring Alex Colgan at Ledger Domain and Herb Wong at Antares Vision Group Watch the Full…

We’ve already explained why visibility can’t wait in our latest blog, Why Cold Chain Visibility Is No Longer Optional for…